Infertility in men
Highlights
Causes of Male Infertility
More than 90% of male infertility cases are due to low sperm counts, poor sperm quality, or both. The remaining cases of male infertility can be caused by a range of conditions including anatomical problems, hormonal imbalances, and genetic defects.
Sperm Abnormalities
Sperm abnormalities are a critical factor in male infertility. These abnormalities include:
- Low sperm count
- Poor sperm motility (movement)
- Abnormal sperm shape
Risk Factors
Risk factors for male infertility include:
- Varicocele, an enlarged varicose vein in the spermatic cord that connects to the testicle
- Aging, which can reduce sperm counts and motility and decrease the genetic quality of sperm
- Sexually transmitted diseases, which can cause scarring in the male reproductive system or impair sperm function
- Lifestyle factors such as smoking and substance abuse
- Long-term or intensive exposure to certain types of chemicals, toxins, or medications
Diagnosis
In addition to a medical history and physical exam, specific tests for male infertility include:
- Semen analysis to evaluate the quantity and quality of sperm
- Blood tests to evaluate hormone levels
- Imaging tests to look for structural problems
- Genetic testing to identify sperm DNA fragmentation, chromosomal defects, or genetic diseases
Treatment
Treatment for male infertility should first address any underlying medical conditions that may be contributing to fertility problems. Drug therapy may be used to treat hormonal disorders. Surgery may be used to repair varicoceles and correct any obstructions in the reproductive tract.
If fertility issues remain unresolved, intracytoplasmic sperm injection (ICSI) is commonly used in combination with in vitro fertilization (IVF) to achieve pregnancy when male infertility is a factor. ICSI involves injecting a single sperm into an egg obtained through IVF. The fertilized egg is then implanted back into the woman. Pregnancy success rates depend on many different factors.
Introduction
Infertility is the failure of a couple to become pregnant after one year of regular, unprotected intercourse. About a third of infertility problems are due to female infertility, and another third are due to male infertility. In the remaining cases, infertility affects both partners or the cause is unclear. [For information about female infertility, see In-Depth Report #22: Infertility in women.]
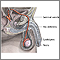
The Male Reproductive System
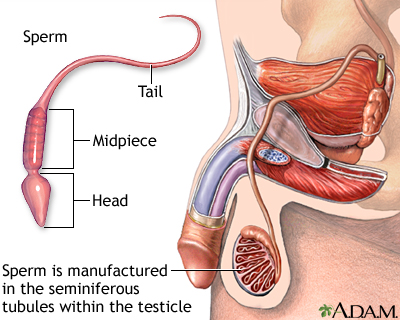
Male fertility depends on the proper function of a complex system of organs and hormones:
- The process begins in the area of the brain called the hypothalamus-pituitary axis, a system of glands, hormones, and chemical messengers called neurotransmitters, all of which are critical for reproduction.
- The first step in fertility is the production of gonadotropin-releasing hormone (GnRH) in the hypothalamus, which prompts the pituitary gland to manufacture follicle-stimulating hormone (FSH) and luteinizing hormone (LH).
- FSH maintains sperm production, and LH stimulates the production of the male hormone testosterone.
- Both sperm and testosterone production occurs in the two testicles, or testes, which are contained in the scrotal sac (the scrotum). (This sac develops on the outside of the body because internal body temperature is too high to allow sperm production.)
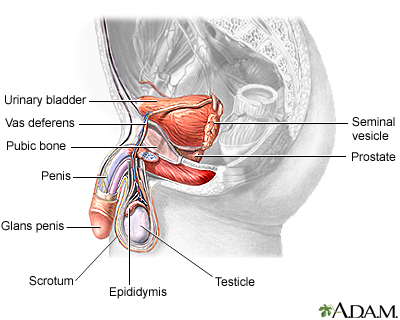
Sperm
Sperm are made in hundreds of microscopic tubes, known as seminiferous tubules, which make up most of the testicles.
Surrounding these tubules are clumps of tissue containing Leydig cells, which produce testosterone when stimulated by luteinizing hormone (LH).
Sperm Development. The life cycle of sperm takes about 74 days:
- Sperm begin partially embedded in nurturing Sertoli cells, which are located in the lower parts of the seminiferous tubules.
- As they mature and move along, they are stored in the upper part of the tubules. Young sperm cells are known as spermatids.
- When the sperm has completed the development of its head and tail, it is released from the cell into the epididymis. This C-shaped tube is 1/300 of an inch in diameter and about 20 feet long. It loops back and forth on itself within a space that is only about one and a half inches long. The sperm's journey through the epididymis takes about 3 weeks.
- The fluid in which the sperm is transported contains sugar in the form of fructose, which provides energy as the sperm matures. In the early stages of its passage, the sperm cannot swim in a forward direction and can only vibrate its tail weakly. By the time the sperm reaches the end of the epididymis, however, it is mature and looks like a microscopic squirming tadpole.
- At maturity, each healthy sperm consists of a head that contains the man's genetic material (his DNA) and a tail that lashes back and forth at great speed to propel the head forward at about four times its own length every second. The ability of a sperm to move forward rapidly and straight is probably the most significant determinant of male fertility.
Ejaculation. When a man experiences sexual excitement, nerves stimulate the muscles in the epididymis to contract, which forces the sperm out through the penis:
- After being produced in the testicle, the sperm first pass through the epididymis and then into one of two rigid and wire-like muscular channels, called the vasa deferentia. (A single member of this pair of channels is called a vas deferens.)
- Muscle contractions in the vas deferens from sexual activity propel the sperm along past the seminal vesicles. These are clusters of tissue that contribute fluid, called seminal fluid, to the sperm. The vas deferens also collects fluid from the nearby prostate gland. This mixture of various fluids and sperm is the semen.
- Each vas deferens then joins together to form the ejaculatory duct. This duct, which now contains the sperm-containing semen, passes down through the urethra. (The urethra is the same channel in the penis through which a man urinates, but during orgasm, muscles close off the bladder so that urine cannot enter the urethra.)
- The semen is forced through the urethra during ejaculation, the final stage of orgasm when the sperm is literally shot out of the penis.
Semen. In addition to providing the fluid that transports the sperm, semen also has other benefits:
- It provides a very short-lived alkaline environment to protect sperm from the harsh acidity of the female vagina. (If the sperm do not reach the woman's cervix within several hours, the semen itself becomes toxic to sperm and they die.)
- It contains a gelatin-like substance that prevents it from draining from the vagina too quickly.
- It contains sugar in the form of fructose to provide instant energy for sperm locomotion.
The Path to the Egg. The sperm's passage to the egg is a difficult journey.
- Usually about 100 - 300 million sperm are delivered into the ejaculate at any given time. Even under normal conditions only about 15% of these millions of sperm are strong enough to fertilize an egg.
- After the stress of ejaculation, only about 400 sperm survive the orgasm to continue the journey.
- Out of this number, only about 40 sperm survive the challenges posed by the semen and the environment of the vagina to reach the vicinity of the egg. Normally, the cervical mucus forms an impenetrable barrier to sperm. However, when a woman ovulates (releases her egg, the oocyte), the mucous lining thins to allow sperm penetration.
- Sperm that manage to reach the mucus lining in the woman's cervix (the lower part of her uterus) must survive about four more days to reach the woman's fallopian tubes. (Here, the egg is positioned for fertilization for only 12 hours each month.)
- The few remaining sperm that penetrate the cervical mucus and are able to reach the fallopian tubes become capacitated.
- Capacitation is a one-time explosion of energy that completes the sperm's journey. It boosts the motion of the sperm and triggers the actions of the acrosome, a membrane that covers the head of the sperm and resembles a warhead. The acrosome is dissolved, and enzymes contained within it are released to allow the sperm to drill a hole through the tough outer coating of the egg.
- In the end, only one sperm gets through to fertilize the egg.
Causes
More than 90% of male infertility cases are due to low sperm counts, poor sperm quality, or both. The remaining cases of male infertility can be caused by a number of factors including anatomical problems, hormonal imbalances, and genetic defects.
Sperm Abnormalities
Sperm abnormalities can be caused by a range of factors, including congenital birth defects, disease, chemical exposure, and lifestyle habits. (See Risk Factors section.) In many cases, the causes of sperm abnormalities are unknown.
Sperm abnormalities are categorized by whether they affect sperm count, sperm movement, or sperm shape. They include:
- Low Sperm Count (Oligospermia). A sperm count of less than 20 million/mL is considered low sperm. Azoospermia refers to the complete absence of sperm cells in the ejaculate. Partial obstruction anywhere in the long passages through which sperm pass can reduce sperm counts. Sperm count varies widely over time, and temporary low counts are common. A single test that reports a low count may not be a representative result.
- Poor Sperm Motility (Asthenospermia). Sperm motility is the sperm's ability to move. If movement is slow or not in a straight line, the sperm have difficulty invading the cervical mucus or penetrating the hard outer shell of the egg. If 60% or more of sperm have normal motility, the sperm is at least average in quality. If less than 40% of sperm are able to move in a straight line, the condition is considered abnormal. Sperm that move sluggishly may have genetic or other defects that render them incapable of fertilizing the egg. Poor sperm motility may be associated with DNA fragmentation and may increase the risk for passing on genetic diseases.
- Abnormal Sperm Morphology (Teratospermia). Morphology refers to shape and structure. Abnormally shaped sperm cannot fertilize an egg. About 60% of the sperm should be normal in size and shape for adequate fertility. The perfect sperm structure is an oval head and long tail.
Retrograde Ejaculation
Retrograde ejaculation occurs when the muscles of the bladder wall do not function properly during orgasm and sperm are forced backward into the bladder instead of forward out of the urethra. Sperm quality is often impaired.
Retrograde ejaculation can result from several conditions:
- Surgery to the lower part of the bladder or prostate (the most common cause of retrograde ejaculation)
- Diseases such as diabetes and multiple sclerosis
- Spinal cord injury or surgery
- Medications such as alpha blockers used for enlarged prostate glands, tranquilizers, certain antipsychotics, or blood pressure medications may also cause temporary retrograde ejaculation.
- Aging
Structural Abnormalities
Any structural abnormalities that damage or block the testes, tubes, or other reproductive structures can affect fertility:
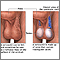
- Cryptorchidism. Cryptorchidism is a condition usually seen in newborn infants in which the testicles fail to descend from the abdomen into the scrotum. Cryptorchidism is associated with mild to severe impairment of sperm production.
- Hypospadias. Hypospadias is a birth defect in which the urinary opening is on the underside of the penis. It can prevent sperm from reaching the cervix if not surgically correct.
- Blockage in the Tubes that Transport Sperm. Some men are born with a blockage or other problems in the epididymis or ejaculatory ducts, that later affect fertility. Some men lack the vas deferens, the tube that carries sperm from the testicles out through the penis. Low semen levels in ejaculate may be associated with structural abnormalities in the tubes transporting the sperm.
Hormonal Deficiencies
Hypogonadism is the general name for a severe deficiency in gonadotropin-releasing hormone (GnRH), the primary hormone that signals the process leading to the release of testosterone and other important reproductive hormones. Low levels of testosterone from any cause may result in defective sperm production.
Hypogonadism is uncommon and is most often present at the time of birth. It is usually the result of rare genetic diseases that affect the pituitary gland. These conditions may include selective deficiencies of the hormones FSH and LH, Kallman syndrome, or panhypopituitarism, in which the pituitary gland fails to make almost all hormones. Hypogonadism can also develop later in life from brain or pituitary gland tumors or as a result of radiation treatments.
Genetic Disorders
Certain inherited disorders can impair fertility. Examples include:
- Cystic fibrosis can cause missing or obstructed vasa deferentia (the tubes that carry sperm).
- Polycystic kidney disease, a relatively common genetic disorder that causes large cysts to form on the kidneys and other organs during adulthood, may cause infertility as the first symptom if cysts develop in the reproductive tract.
- Klinefelter syndrome is marked by two X and one Y chromosomes (the norm is one X and one Y), which causes low testosterone levels and abnormalities of the seminiferous tubules, although most other male physical attributes are normal.
- Kartagener syndrome is a rare disorder that causes impaired sperm motility as well as severe respiratory infections and a reversed position of the major organs.
Risk Factors
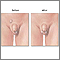
Varicocele
A varicocele is an abnormally enlarged and twisted (varicose) vein in the spermatic cord that connects to the testicle. Varicoceles are found in about 15% of all men and in about 40% of infertile men, although it is not clear how much they affect fertility or by what mechanisms. They can raise testicular temperature, which may have effects on sperm production, movement, and shape.
Age
Age-related sperm changes in men are not abrupt, but are a gradual process. Aging can adversely affect sperm counts and sperm motility (the sperm's ability to swim quickly and move in a straight line). The genetic quality of sperm declines as a man ages.
Sexually Transmitted Diseases
Repeated Chlamydia trachomatis or gonorrhea infections are the sexually transmitted diseases most often associated with male infertility. Such infections can cause scarring and block sperm passage. Human papillomaviruses, the cause of genital warts, may also impair sperm function.
Lifestyle Factors
Nearly any major physical or mental stress can temporarily reduce sperm count. Some common conditions that lower sperm count, temporarily in nearly all cases, include:
- Testicular Overheating. Overheating, such as from high fevers, saunas, and hot tubs, may temporarily lower sperm count.
- Substance Abuse. Cocaine or heavy marijuana use can temporarily reduce the number and quality of sperm. Chemical compounds in marijuana may impair sperms' ability to swim and also inhibit their ability to penetrate the egg. Anabolic steroid use can shrink testicles and decrease sperm production. Heavy drinking may also impair fertility.
- Smoking. Cigarette smoking may affect sperm quality.
- Obesity. Obesity may impair hormonal levels and adversely affect fertility.
- Bicycling. Prolonged bicycling may affect erectile function. Pressure from the bike seat can sometimes damage blood vessels and nerves that are responsible for erections. Mountain biking, which involves riding on off-road terrain, exposes the perineum (the region between the scrotum and the anus) to more extreme shocks and vibrations and increases the risk for injuries to the scrotum. A padded or contoured bike seat set at the proper height and angle can help reduce this risk.
- Emotional Stress. Stress may interfere with certain hormones involved with sperm production but doctors are not sure if stress plays an important role in infertility.
Environmental Factors
Occupational or other long-term exposure to certain types of toxins and chemicals (such as herbicides and pesticides) may reduce sperm count by either affecting testicular function or altering hormone systems. Estrogen-like and hormone-disrupting chemicals such as bisphenol A, phthalates, and organochlorines are particular potential concerns. Chronic exposure to heavy metals such as lead, cadmium, or arsenic may affect sperm quality. These chemicals generally affect men who have long-term and intense occupational exposure to them. At this time, there is no strong evidence supporting a serious harmful effect on fertility in men who have normal limited exposure to these chemicals.
Medical Conditions
Medical conditions that can affect male fertility include any severe injury or major surgery, diabetes, HIV, thyroid disease, Cushing syndrome, heart attack, liver or kidney failure, and chronic anemia. Certain types of medications can impair sperm production.
Infections in the Urinary Tract or Genitals. Infections that may affect fertility include prostatitis (inflammation in the prostate gland), orchitis (in the testicle), semino-vesculitis (in the glands that produce semen), or urethritis (in the urethra), perhaps by altering sperm motility. Even after successful antibiotic treatment, infections in the testes may leave scar tissue that blocks the epididymis.
Cancer and Its Treatments. Cancer treatments such as chemotherapy and radiation can damage sperm quality and quantity, causing infertility. The closer radiation treatments are to reproductive organs, the higher the risk for infertility. There is also some evidence that male infertility is itself a risk factor for testicular cancer.
Diagnosis
In any fertility work-up, both male and female partners are tested if pregnancy fails to occur after a year of regular unprotected sexual intercourse. It should be done earlier if a woman is over age 35 or if either partner has known risk factors for infertility. A work-up can not only uncover the causes of infertility but also detect other potentially serious medical problems, including genetic mutations, cancer, or diabetes.
Fertility History
The doctor will ask about any medical or sexual factors that might affect fertility:
- Frequency and timing of sexual intercourse
- Duration of infertility and any previous fertility events
- Childhood illnesses and any problems in development
- Any serious illness (such as diabetes, respiratory infections, cancer, or previous surgeries)
- Sexual history, including any sexually transmitted diseases
- Any exposure to toxins, such as chemicals or radiation
- History of any medications and allergies
- Any family history of reproductive problems
Physical Exam
A fertility specialist, usually a urologist, will perform a physical examination. A physical examination of the scrotum, including the testes, is essential for any male fertility work-up. It is useful for detecting large varicoceles, undescended testes, absence of vas deferens, cysts, or other physical abnormalities.
- Varicoceles large enough to possibly interfere with fertility can be felt during examination of the scrotum. In such cases, they are described as feeling like "a bag of worms." They disappear or are greatly reduced when the patient lies down, so the patient should be examined for varicocele while standing.
- Checking the size of the testicles is helpful. Smaller-sized and softer testicles along with tests that show low sperm count are strongly associated with problems in sperm formation. Normal testicles accompanied by a low sperm count, however, suggest possible obstruction. The doctor may also take the temperature of the scrotum with a test called scrotal thermography.
- The doctor will also check the prostate gland for abnormalities.
- The penis is checked for warts, discharge from the urinary tract, and hypospadias (incorrect location of the urethra opening).
Post-Ejaculatory Urine Sample
A urine sample to detect sperm after ejaculation may rule out or indicate retrograde ejaculation. It also may be used to test for infections.
Semen Analysis
The basic test to evaluate a man's fertility is a semen analysis. The sperm collection test for men who can produce semen involves the following steps:
- A man should abstain from ejaculation for several days before the test because each ejaculation can reduce the number of sperm by as much as a third. To ensure an accurate sample, most doctors recommend abstaining from ejaculation for at least 2 days, but not more than 5 days, prior to semen collection.
- A man collects a sample of his semen in a collection jar during masturbation either at home or at the doctor's office. Proper collection procedure is important, since the highest concentration of sperm is contained in the initial portion of the ejaculate. Specially designed condoms are also available that enable collection of a sample during sexual intercourse. (Regular condoms are not useful, since they often contain substances that kill sperm.)
- The sample should be kept at body temperature and delivered promptly. If the sperm are not analyzed within 2 hours or kept reasonably warm, a large proportion may die or lose motility.
- A semen analysis should be repeated at least three times over several months.
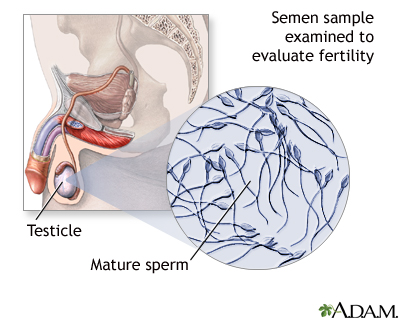
The man and woman should both be present when the doctor discusses the results of this analysis so that both partners understand the implications. The analysis report should contain results of any abnormalities in sperm count, motility, and morphology as well as any problem in the semen. However, semen analysis alone is not necessarily a definitive indicator of either infertility or fertility.
A semen analysis will provide information on:
- Amount of semen produced (volume)
- Number of sperm per milliliter of semen (concentration)
- Total number of sperm in the sample (count)
- Percentage of moving sperm (motility)
- Shape of sperm (morphology)
Semen Volume and Concentration. The seminal fluid (semen) itself is analyzed for abnormalities. The color is checked and should be whitish-gray.
The amount of semen is important. Most men ejaculate 2.5 - 5 milliliters (mL) (1/2 - 1 teaspoon) of semen. Either significantly higher or lower amounts can be a sign of prostate problems, blockage, or retrograde ejaculation.
The semen will be tested for how liquid it is. Abnormal results may suggest prostate gland problems or lack of sperm.
Other factors may also be measured:
- An absence of semen fructose (sugar) may indicate obstruction in the vas deferens or epididymis.
- Low levels of a substance called inhibin B, which is produced only in the testes, may indicate blockage or other defects in the seminiferous tubules.
- Low levels of another compound, alpha-glucosidase, may also indicate blockage in the epididymis.
Sperm Count. A low sperm count should not be viewed as a definitive diagnosis of infertility but rather as one indicator of a fertility problem. In general, a normal sperm count is considered to be 20 million per milliliter of semen.
Sperm Motility. Motility (the speed and quality of movement) is graded on a 1 - 4 ranking system. For fertility, motility should be greater than 2.
- Grade 1 sperm wriggle sluggishly and make little forward progress. (Sperm that, in fact, clump together may indicate that antibodies to the sperm are present.)
- Grade 2 sperm move forward, but they are either very slow or do not move in a straight line.
- Grade 3 sperm move in a straight line at a reasonable speed and can home in on an egg accurately.
- Grade 4 sperm are as accurate as Grade 3 sperm, but move at a very rapid speed.
More than 63% of sperm should be motile for normal fertility, but even men whose motile sperm constitutes only about a third of the total sperm count should not rule out conception. Testing for sperm motility is important for predicting the success of assisted reproductive technologies and which men might be candidates for the intracytoplasmic sperm injection (ICSI) fertilization technique, in which the sperm is inserted directly into the egg and motility plays almost no role.
Sperm Morphology. Morphology is the shape and structure of the sperm. Determining the morphology of the sperm is particularly important for the success of the fertility treatments in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI).
Blood Tests
Blood tests are used for measuring several factors that might affect fertility:
Hormonal Levels. Men produce both male hormones (testosterone) as well as pituitary hormones (FSH and LH). Tests for these hormone levels are indicated if semen analysis is abnormal (especially if sperm concentration is less than 10 million per milliliter) or there are other indications of hormonal disorders.
- Blood tests for testosterone and follicle-stimulating hormone (FSH) levels are usually taken first.
- If testosterone levels are low, then luteinizing hormone (LH) is measured.
Low levels of FSH, LH, and testosterone may indicate a diagnosis of hypogonadotropic hypogonadism. Very high FSH levels with normal levels of other hormones indicate abnormalities in initial sperm production. Usually this occurs only if the testicles are severely defective, causing Sertoli cell-only syndrome, in which sperm-manufacturing cells are absent. Other hormones, such as prolactin, estrogen, or stress hormones may be measured if there are symptoms of other problems, such as low sexual drive or the presence of breasts.
Infections. Blood tests can determine the presence of any infections that might affect fertility, including HIV, hepatitis, and Chlamydia.
Ultrasound
Ultrasound imaging may be used to accurately determine the size of the testes or to detect cysts, tumors, abnormal blood flow, or varicoceles that are too small for physical detection (although such small veins may have little or no effect on fertility). It can also help detect testicular cancer.
Sperm Penetration Tests
Cervical Mucus Penetration Test. This post-coital test is designed to evaluate the effect of a woman's cervical mucus on a man's sperm. Typically, a woman is asked to come into the doctor's office within 2 - 24 hours after intercourse at mid-cycle (when ovulation should occur). A small sample of her cervical mucus is examined under a microscope. If the doctor observes no surviving sperm or no sperm at all, the cervical mucus will then be cultured for the presence of infection. The test cannot evaluate sperm movement from the cervix into the fallopian tubes or the sperm's ability to fertilize an egg.
Micro-Penetration Assay Test. This test checks to see if sperm can penetrate hamster eggs that have had their covering removed. If fewer than 5 - 20% of the eggs are penetrated, infertility is diagnosed. It may be useful for determining the best assisted reproductive treatment options for men with infertility.
Genetic Testing
Genetic testing may be warranted in men who are severely deficient in sperm and who show no evidence of obstruction, particularly in men undergoing the intracytoplasmic sperm injection (ICSI) procedure. Genetic testing can help identify sperm DNA fragmentation, chromosomal defects, or the possibility of genetic diseases that can be passed on to children. If genetic abnormalities are suspected in either partner, counseling is recommended.
Treatment
Treatment for infertility should first address any underlying medical conditions that may be contributing to fertility problems. Drug therapy may be used to treat hypogonadism and other hormonally related conditions. Surgery is used to repair varicoceles and correct any obstructions in the reproductive tract. However, there is some controversy over whether varicocele embolisation or surgery actually improves fertility. Some studies indicate that varicocele treatment may not help improve a couple’s chances of conception.
If fertility issues remain unresolved, intrauterine insemination (also called artificial insemination) and assisted reproductive technologies such as in vitro fertilization should be considered. Intracytoplasmic sperm injection is commonly used in combination with in vitro fertilization in cases of male factor infertility. [See Assisted Reproductive Technologies section in this report.] The couple can also discuss with a fertility specialist other options such as donor sperm or eggs.
Choosing a Fertility Clinic
Choosing a good fertility clinic is important. Those offering assisted reproductive techniques are not always regulated by the government, and abuses have been reported, including lack of informed consent, unauthorized use of embryos, and failure to routinely screen donors for disease.
The clinic should always provide the following information:
- The live-birth rate (not just pregnancy success rate) for other couples with similar infertility problems. (Multiple births, such as twins or triplets, are counted as one live birth.)
- Such statistics should include high-risk women, such as those who are older or fail to produce eggs. (Some disreputable clinics give success percentages that exclude high-risk women from their total, thereby making the percentage of success much higher.)
Special Considerations for Patients with Cancer
Adolescents and adult men undergoing cancer treatments who may want to father children in the future should consider banking and freezing their sperm for later use in assisted reproductive therapies. This technique is called sperm cryopreservation. Sperm cryopreservation is recommended by the American Society of Clinical Oncology as the method with the highest likelihood of success for male cancer survivors. However, these banking methods are not appropriate for pre-adolescent boys being treated for childhood cancers such as leukemia. Researchers are investigating ways that stem cell transplantation may someday help these children regain their fertility while avoiding leukemia relapse.
Assisted Reproductive Technologies
Assisted reproductive technologies (ART) are medical techniques that help couples conceive. These procedures involve either:
- A couple's own eggs or sperm
- Donor eggs, sperm, or embryos
Fertilization may occur either in the laboratory or in the uterus. In the U.S., the number of live birth deliveries from ART has dramatically increased in the last decade. About 40,000 live births (deliveries of one or more infants) occur in the U.S. each year using assisted reproductive technologies.
Technically, the term ART refers only to fertility treatments, such as in vitro fertilization (IVF) and its variants, which handle both egg and sperm. Therefore intrauterine insemination (artificial insemination) is not officially considered a form of ART.
Sperm Retrieval Procedures
Before fertilization using intrauterine insemination (IUI) or intracytoplasmic sperm injection (ICSI) can take place, the sperm must be collected and prepared.
When a man has no available sperm in the ejaculate (usually from blockage, vasectomy, or lack of vas deferens), the sperm must be retrieved from the testes or the epididymis. Various microsurgical techniques are used for retrieval. The procedure may be done under local or general anesthesia, using a spring-loaded biopsy device, a thin needle, incisions, or microsurgical techniques. Most procedures can be done on an outpatient basis, and the patient returns home the same day. There is no conclusive evidence that one procedure works better than another.
Testicular Fine Needle Aspiration. With testicular fine needle aspiration (TFNA), the surgeon uses a fine needle to remove sperm. This can be performed with local anesthetic and by surgeons who do not have experience in microsurgeries.
Microsurgical Epididymal Sperm Aspiration. Microsurgical epididymal sperm aspiration (MESA) uses microsurgical techniques to collect sperm that are close to blocked portions of the epididymis. It involves an open incision and may be done under general or spinal anesthesia in a hospital setting, although the patient can often go home the same day. The doctor accesses the epididymis and retrieves sperm with an extremely fine needle-like device. It has the advantage that it can retrieve the largest number of sperm compared to other procedures. However, as with any invasive procedure, it carries some risks of complications, such as bleeding or infection.
Percutaneous Epididymal Sperm Aspiration. Percutaneous epididymal sperm aspiration (PESA) uses a needle to obtain mature sperm from areas in the upper parts of the epididymis (the coiled tube where sperm are stored before ejaculation). It is performed under local anesthesia, sometimes in the doctor's office, is less expensive than other techniques, and recovery is fairly painless. However, it has less of a chance of achieving sufficient sperm than MESA, and there is also a chance of hitting a blood vessel, causing bleeding.
Testicular Sperm Extraction. Testicular sperm extraction (TESE) is a microsurgery that removes a small amount of tissue from one or more areas of the testes using incisions and microsurgery techniques. The tissue is placed in a culture and chopped into tiny pieces, and the sperm are extracted. It is a complex process, however, and may cause more pain than other sperm retrieval procedures.
Testicular Sperm Aspiration. Testicular sperm aspiration (TESA) uses a needle-like biopsy device to draw a small sample of testicular tissue. Multiple attempts are sometimes required to retrieve sperm.
Sperm Washing
Sperm washing is done to prepare the sperm for use in ART procedures. Methods for washing sperm can help remove chemicals (prostaglandins) that can cause the woman’s uterus to contract and cramp. Sperm washing can also help remove sexually transmitted viruses, such as HIV and hepatitis, which could potentially be transmitted to the woman during fertility treatment. There are three basic methods for sperm washing:
- Simple sperm wash dilutes sperm in a test tube and then uses a centrifuge to retrieve sperm cells
- Density gradient sperm wash is similar to the simple sperm wash but is better for separating dead sperm from live, healthy sperm cells
- Swim-up technique does not use a centrifuge. Instead, it uses a culture dish with a medium that attracts sperm powerful enough to swim up to the nutrient mixture. This technique is useful for harvesting healthy sperm with good motility. It is not helpful for men who have low sperm counts or poorly motile sperm.
Freezing Sperm
Sperm can be fresh or frozen in advance. Frozen sperm provide excellent results for fertilization procedures. Fresh sperm, however, are preferred by some centers for cases when low sperm count is not caused by obstruction.
Intrauterine Insemination (Artificial Insemination)
Artificial insemination (AI) is the least complex of fertility procedures and is often tried first in uncomplicated cases of infertility. AI involves placing the sperm directly in the cervix (called intracervical insemination) or into the uterus (called intrauterine insemination, or IUI). IUI is the standard AI procedure.
Intrauterine insemination may be used under the following circumstances.
- When the woman's cervical mucus is unreceptive.
- When donor sperm are required.
- If the man's sperm count is very low.
- If there is decreased sperm motility.
- When unexplained infertility exists in both partners.
The intrauterine insemination procedure is as follows:
- A woman usually takes fertility drugs in advance.
- The man must produce sperm at the time the woman is ovulating.
- The sperm are subjected to certain so-called "washing" procedures. They are then inserted into the uterine cavity through a long, thin catheter.
The administration of fertility drugs and sperm retrieval is timed so that the process can coincide with time of ovulation.
If a woman fails to conceive after IUI, she may be a candidate for in vitro fertilization (IVF). [For more information on intrauterine insemination, see In-Depth Report #22: Infertility in women.]
Intracytoplasmic Sperm Injection (ICSI)
Intracytoplasmic sperm injection (ICSI) is an assisted reproductive technology (ART) used for couples when male infertility is the main factor. It is used in combination with in vitro fertilization (IVF). It involves injecting a single sperm into an egg obtained from IVF. [For more information, see In-Depth Report #22: Infertility in women.]
The procedure is very simple:
- A tiny glass tube (called a holding pipet) stabilizes the egg.
- A second glass tube (called the injection pipet) is used to penetrate the egg's membrane and deposit a single sperm into the egg.
- The egg is released into a drop of cultured medium.
- If fertilized, the egg is allowed to develop for 1 - 2 days and then is either frozen or implanted.
The greatest concern with this procedure has been whether it increases the risk for birth defects. Many, but not all, studies have reported no higher risks of birth defects in children born using ICSI procedures. However, if the father’s infertility was due to genetic issues, this genetic defect may be passed on to male children conceived through ICSI.
Because several embryos are implanted to increase the chances for pregnancy success, multiple births are frequently an outcome of IVF/ICSI. Multiple pregnancies increase the risks for a mother and her babies. In particular, there is increased risk for premature delivery and low birth weight. These factors can cause heart and lung problems and developmental disabilities in children.
IVF/ICSI can also pose specific risks for the woman. These risks include ovarian hyperstimulation, a condition induced by the fertility drugs used in the procedure. Ovarian hyperstimulation can result in dangerous fluid and electrolyte imbalances as well as increased blood pressure and higher risk for blood clots.
Another concern has been whether the ICSI procedure is overused. ICSI use has increased 5-fold over the past decade and is now used in most ART procedures, even though the proportion of men receiving treatment for male infertility has remained the same. Some doctors recommend ICSI for women who have failed prior IVF attempts or who have few or poor-quality eggs, even if their male partners have normal semen measurements. According to the Society for Assisted Reproductive Technology, there is little evidence that ICSI helps improve pregnancy success for couples who do not have a problem with male factor infertility.
Success Rates. Not all IVF/ICSI cycles result in pregnancy, and not all IVF-achieved pregnancies result in live births. When the woman’s own eggs are used, results are better with fresh embryos than frozen embryos. Success rates provided by fertility clinics are not always a reliable indicator as they depend on many variables, including the age of the patients. The chances for success are best for women younger than age 37.
Lifestyle Changes
Timing and Monitoring Sexual Activity for Best Results
Both male and female hormone levels fluctuate according to the time of day, and they also vary from day to day and month to month. Some timing tips might be helpful.
Fertility and Seasonal Changes. Some studies have reported higher sperm counts in the winter than in the summer. For women, fertility rates as measured by treatment success are highest in months when days are longest.
Monitoring Basal Body Temperature. To determine the most likely time of ovulation and therefore the time of fertility, a woman should take her body temperature, called her basal body temperature. This is the body's temperature as it rises and falls in accord with hormonal fluctuations.
By studying the temperature patterns after a few months, couples can begin to anticipate ovulation and plan their sexual activity accordingly.
Frequency of Intercourse. It is not clear how often a couple should have intercourse in order to conceive. Some doctors think that having sex more than 2 days a week adds no benefits. In addition, frequent sexual activity lowers sperm count per ejaculation. Some studies have indicated, however, that having intercourse every day, or even several times a day, before and during ovulation, improves pregnancy rates. Although sperm count per ejaculation is low, a constantly replenished semen supply is more likely to result in a fertilized egg.
Dietary Considerations
Everyone should eat a healthy diet rich in fresh fruits, vegetables, and whole grains. Replace animal fats with monounsaturated oils, such as olive oil. Certain specific nutrients and vitamins have been studied for their effects on male infertility and sperm health. They include antioxidant vitamins (vitamin C, vitamin E) and the dietary supplements L-carnitine and L-acetylcarnitine. To date, there is no conclusive evidence that they are effective.
Other Lifestyle Changes
Other tips for helping fertility include:
- Avoid cigarettes and any drugs that may affect sperm count or reduce sexual function.
- Overweight men should try to reduce their weight as obesity may be associated with infertility.
- Get sufficient rest, and exercise moderately but regularly. (Excessive exercise can impair fertility.)
- Stress may contribute to reduced sperm quality. It is not known if stress reduction techniques can improve fertility, but they may help couples endure the difficult processes involved in fertility treatments.
- Although studies indicate that tight underwear and pants pose no threat to male fertility, there is no harm in wearing looser clothing.
- To prevent overheating of the testes, men should avoid hot baths, showers, and steam rooms.
- Avoid use of sexual lubricants (Astroglide, KY-jelly) as they may affect sperm motility.
Dealing with Stress
The fertility treatment process presents a roller coaster of emotions. There are almost no sure ways to predict which couples will eventually conceive. Some couples with multiple problems will overcome great odds, while other, seemingly fertile, couples fail to conceive. Many of the new treatments are remarkable, but a live birth is never guaranteed. The emotional burden on the couple is considerable, and some planning is helpful:
- Decide in advance how many and what kind of procedures will be emotionally and financially acceptable and attempt to determine a final limit. Fertility treatments are expensive. A successful pregnancy often depends on repeated attempts.
- Prepare for multiple births as a possible outcome for successful pregnancy (especially if assisted reproductive technologies are used). A pregnancy that results in a multiple birth introduces new complexities and emotional problems.
- Determine alternatives (adoption, donor sperm or egg, or having no children) as early as possible in the fertility process. This can reduce anxiety during treatments and feelings of disappointment if conception does not occur.
Resources
- www.asrm.org -- American Society for Reproductive Medicine
- www.urologyhealth.org -- American Urological Association
- www.theafa.org -- American Fertility Association
- www.sart.org -- Society for Assisted Reproductive Technology
- www.cdc.gov/ART/ -- Centers for Disease Control: Assisted Reproductive Technology Report
References
American Urological Association. The Optimal Evaluation of the Infertile Male. AUA Best Practice Statement. Revised 2010.
Bensdorp AJ, Cohlen BJ, Heineman MJ, Vandekerckhove P. Intra-uterine insemination for male subfertility. Cochrane Database Syst Rev. 2007 Oct 17;(4):CD000360.
Davies MJ, Moore VM, Willson KJ, Van Essen P, Priest K, Scott H, et al. Reproductive technologies and the risk of birth defects. N Engl J Med. 2012 May 10;366(19):1803-13. Epub 2012 May 5.
Evers JH, Collins J, Clarke J. Surgery or embolisation for varicoceles in subfertile men. Cochrane Database Syst Rev. 2009 Jan 21;(1):CD000479.
Hammoud AO, Gibson M, Peterson CM, Meikle AW, Carrell DT. Impact of male obesity on infertility: a critical review of the current literature. Fertil Steril. 2008 Oct;90(4):897-904
Jain T, Gupta RS. Trends in the use of intracytoplasmic sperm injection in the United States. N Engl J Med. 2007 Jul 19;357(3):251-7.
Khera M, Lipshultz LI. Evolving approach to the varicocele. Urol Clin North Am. 2008 May;35(2):183-9, viii.
Levine BA, Grifo JA. Intrauterine insemination and male subfertility. Urol Clin North Am. 2008 May;35(2):271-6.
Practice Committee of American Society for Reproductive Medicine. Guidelines for reducing the risk of viral transmission during fertility treatment. Fertil Steril. 2008 Nov;90(5 Suppl):S156-62.
Showell MG, Brown J, Yazdani A, Stankiewicz MT, Hart RJ. Antioxidants for male subfertility. Cochrane Database Syst Rev. 2011 Jan 19;(1):CD007411.
Van Peperstraten A, Proctor ML, Johnson NP, Philipson G. Techniques for surgical retrieval of sperm prior to intra-cytoplasmic sperm injection (ICSI) for azoospermia. Cochrane Database Syst Rev. 2008 Apr 16;(2):CD002807.
Walsh TJ, Croughan MS, Schembri M, Chan JM, Turek PJ. Increased risk of testicular germ cell cancer among infertile men. Arch Intern Med. 2009 Feb 23;169(4):351-6.
Weedin JW, Khera M, Lipshultz LI. Varicocele repair in patients with nonobstructive azoospermia: a meta-analysis. J Urol. 2010 Jun;183(6):2309-15. Epub 2010 Apr 18.
Zegers-Hochschild F, Adamson GD, de Mouzon J, Ishihara O, Mansour R, Nygren K, et al. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril. 2009 Nov;92(5):1520-4. Epub 2009 Oct 14.
Zhu JL, Basso O, Obel C, Bille C, Olsen J. Infertility, infertility treatment, and congenital malformations: Danish national birth cohort. BMJ. 2006 Sep 30;333(7570):679. Epub 2006 Aug 7.
|
Review Date:
12/17/2012 Reviewed By: Harvey Simon, MD, Editor-in-Chief, Associate Professor of Medicine, Harvard Medical School; Physician, Massachusetts General Hospital. Also reviewed by David Zieve, MD, MHA, Medical Director, A.D.A.M., Inc. |