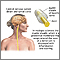
Multiple sclerosis
Highlights
What Is Multiple Sclerosis?
- Multiple sclerosis (MS) is a chronic disease that affects the central nervous system. MS is thought to be an autoimmune disease. In MS, the body’s immune system produces cells and proteins (antibodies) that attack myelin, a fatty substance that protects nerve fibers. The cause of MS is unknown. It is not an inherited disease, but it appears that genetic factors play a role in making some people more susceptible to developing it.
- MS affects significantly more women than men. Most patients first begin to have symptoms between the ages of 20 - 50.
- The course of MS varies among patients. The disease may be mild, moderate, or severe. Most patients have the relapsing-remitting form of MS in which flare-ups (also called relapses or exacerbations) of symptoms are followed by periods of remission.
- Symptoms of MS include fatigue; vision problems; difficulty walking; muscle weakness, stiffness, and spasms; and bladder and bowel problems. Not all patients have all symptoms.
Treatment
Patients with multiple sclerosis are treated with medications and rehabilitation. Seven disease-modifying drugs are approved to treat multiple sclerosis. These drugs can help reduce the frequency and severity of relapses and slow disease progression and disability. Drugs approved by the Food and Drug Administration (FDA) are:
- Interferon beta-1b (Betaseron, Extavia)
- Interferon beta-1a (Avonex)
- Interferon beta-1a (Rebif)
- Glatiramer acetate (Copaxone)
- Natalizumab (Tysabri)
- Mitoxantrone (Novantrone)
- Fingolimod (Gilenya)
- Teriflunomide (Aubagio)
Drug Approvals
- In 2012, the FDA approved teriflunomide (Aubagio), the second oral treatment for relapsing forms of MS. Like fingolimod, teriflunomide is taken as a once-daily pill.
- Botox is now approved for the treatment of urinary incontinence in patients with multiple sclerosis and certain other neurological conditions.
Recent FDA Warnings
In 2012, the FDA warned:
- Fingolimod (Gilenya) should not be used by patients with certain heart conditions
- Dalfampridine (Ampyra) may increase the risk for seizures
- “Liberation therapy” is an unproven and unsafe experimental surgical treatment for multiple sclerosis
.
Introduction
Multiple sclerosis (MS) is a neurological disease that involves the central nervous system (CNS), the nerves that comprise the brain and spinal cord. It has two major features:
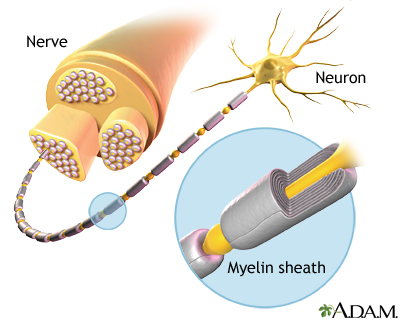
- Destruction of myelin, a fatty insulation covering the nerve fibers, is the main characteristic of MS. The end results of this process, called demyelination, are multiple patches of hard, scarred tissue called plaques or lesions. Sclerosis comes from the Greek word skleros, which means hard.
- Damage of axons, the fibers that carry electric impulses away from a nerve cell, is also a major factor in the permanent disability that occurs with MS.
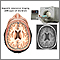
The symptoms, severity, and course of MS vary widely depending partly on the sites of the plaques and the extent of the demyelination.
Autoimmune Disease Process
Multiple sclerosis is thought to be an autoimmune disorder. In autoimmune diseases, immune factors attack the body’s own cells. In the case of MS, the immune system attacks the tissues that make up myelin. The damage to myelin, and nerve fibers (axons), is caused by over-active T cells. T cells are a type of white blood cell called lymphocytes.
Types of Multiple Sclerosis
Doctors generally group multiple sclerosis into four major disease course categories:
- Relapsing-Remitting MS. Relapse-remitting MS (RMSS) is the most common form of multiple sclerosis. About 85% of patients are first diagnosed with this type of MS. RMSS is marked by flare-ups (also called relapses or exacerbations) of symptoms followed by periods of remission when symptoms improve or disappear.
- Secondary-Progressive MS. Some patients with RMSS go on to develop secondary-progressive (SPMS). (For many patients, treatment with disease-modifying medications helps delay this progression.) In SPMS, the disease course continues to worsen with or without periods of remission or leveling off of symptom severity (plateaus).
- Primary-Progressive MS. About 10% of patients are diagnosed with primary-progressive MS (PPMS). In PPMS, symptoms continue to worsen gradually from the very beginning. PPMS has no relapses or remissions. There may be periods of occasional plateaus. This type of multiple sclerosis is more resistant to the medications typically used to treat the illness.
- Progressive-Relapsing MS. Progressive-relapsing MS (PRMS) is a rare form of MS, occurring in less than 5% of patients. It is progressive from the start with intermittent flare-ups of worsening symptoms along the way. There are no periods of remission.
Causes
As with other autoimmune disorders, the exact cause of MS is unknown. A combination of environmental and genetic factors likely plays a role.
Genetic Factors
Multiple sclerosis is not hereditary, but genetic factors appear to play a role in making some people susceptible to the disease process leading to the condition. The most significant genetic link to MS occurs in the major histocompatability complex (MHC), a cluster of genes on chromosome 6 that are essential for immune system function. A much smaller percentage of MS cases may be due to variations in interleukin-7 (IL-7) and interleukin-2 (IL-2) gene receptors, which are also related to immune system regulation.
Environmental Factors
Multiple sclerosis is more common in certain geographical areas of the world, particularly areas that are farther from the equator. Prevalence is generally highest in northern European and North American countries. The clustering of MS cases in these regions has led researchers to investigate whether certain toxins, infections, or vitamin deficiencies (such as vitamin D) may play a factor in triggering MS in genetically susceptible people.
Infectious organisms, mainly viruses, have long been suspects. They include Epstein-Barr virus (the cause of mononucleosis), herpesvirus 6, herpes simplex virus, influenza, measles, mumps, varicella-zoster virus, cytomegalovirus, respiratory syncytial virus, and Chlamydia pneumoniae. However, no direct link has been proven between these infections and multiple sclerosis. There is no evidence that any type of vaccination causes multiple sclerosis.
Risk Factors
About 400,000 Americans and 2.5 million people worldwide suffer from MS.
Age
MS usually first appears between the ages of 20 and 50, with an average age of about 30. It rarely develops before age 15 or after age 60.
Gender
MS is about 2.5 times more common among women than men. The gender gap is strongest among people who develop MS at a younger age. However, some research indicates that men may be more disabled by the disease than women.
Race and Ethnicity
Multiple sclerosis occurs worldwide but is most common in Caucasian people of northern European origin, especially those of Scottish descent.
Family History
A family history of the disease may put some people at risk for MS, although the risk for someone inheriting all the genetic factors associated with MS is only about 2 - 4%. Some research indicates that family members who have MS tend to develop the disease at around the same age. However, family history does not predict whether one family member will experience the same disease severity as another family member.
Possible Protective Factors
Estrogen and Oral Contraceptives. Higher estrogen levels may temporarily lower the risk of developing multiple sclerosis. Studies indicate that oral contraceptives (which contain estrogen) and pregnancy delay the onset of multiple sclerosis. The risk for a first clinical attack increases, however, in the first 6 months after a woman gives birth.
Prognosis
Multiple sclerosis is not a fatal disease. Except in rare cases of severe disease, most people with multiple sclerosis have a normal or near-normal life span and usually die from the same conditions (heart disease or cancer, for example) that affect the general population. Still, MS symptoms can negatively affect quality of life. Suicide rates among patients with MS are higher than average.
The majority of patients with MS do not become severely disabled. Twenty years after diagnosis, about two-thirds of people with MS remain ambulatory and do not need a wheelchair, although many of them may use a cane or crutches for walking assistance. Some patients use an electric scooter or wheelchair to help cope with fatigue or balance problems.
The severity of the disease, and how the disease progresses, varies widely from patient to patient and is unpredictable. About 20% of patients remain asymptomatic or become only mildly symptomatic after an initial clinical event. Another 20% experience a rapidly progressive condition. Most patients will have some degree of disease progression.
Women tend to have a better outlook than men. Factors that determine a higher risk for a severe condition include:
- Age over 40 years at the time of onset of symptoms
- Initial symptoms that affect motor control, mental functioning, or urinary control
- Initial symptoms that affect multiple regions of the body
- Attacks in the first years that are frequent, or a short time between the first two attacks
- Incomplete remissions
- Rapid progression to disability
- MS that is progressive from the beginning or becomes progressive shortly after the onset
Symptoms
Symptoms of multiple sclerosis appear in a variety of ways. Most patients first have a single attack of symptoms, a neurological episode called a clinical isolated syndrome, which typically occurs between the ages of 20 and 50. Initial symptoms may be mild enough that patients do not always seek medical care. Once a second attack occurs, the patient is considered to have relapsing-remitting multiple sclerosis. Much less commonly, the disease is progressive from the start, with the patient having more or less continuous symptoms.
Symptoms in multiple sclerosis depend on the location of the nerve lesion. Not all symptoms affect all patients.
Early Symptoms
Symptoms more likely to occur earlier in the disease include:
- Vision Problems. Optic neuritis, inflammation of the nerves in the eye, is a common early symptom. Patients may initially experience blurred or double vision, usually because of problems with one eye. As the condition progresses, vision loss increases, although total blindness is rare.
- Tingling and Numbness Sensations. Tingling, crawling or burning sensations, or loss of sensation can occur. Patients may feel sensations of intense heat or cold. Symptoms often begin at the end of the legs or arms and move up towards the beginning of the limb. L’Hermmitte’s sign, which is caused by lesions in the cervical spine in the neck, is an electrical buzzing sensation that runs down the back and into the legs. It occurs when bending the neck forward.
- Muscle Weakness and Spasms. Patients can feel weakness, clumsiness, or heaviness in the limbs. They may have difficulty with finger dexterity. Muscle spasms and stiffness (spasticity), particularly in the legs, occur in an initial attack of MS in nearly half of patients.
- Problems with Balance and Coordination. Patients have an unsteady gait and difficulty walking normally and keeping their balance. They may have trouble grasping small objects. These problems can be compounded by other common MS symptoms, such as dizziness and tremor. Ataxia (lack of muscle coordination) and tremors (shaking or trembling of limb) affect up to half of patients.
- Fatigue. Fatigue is the most common and debilitating symptom of MS and often occurs early in the disease. Fatigue is typically worse in the late afternoon and improves in the early evening, and may be accompanied by an increase in body temperature. At the onset, fatigue occurs in about 20% of patients, but as the disease progresses, this is a significant symptom in nearly all patients.
Other Common Symptoms
Other common symptoms that progress over time include:
Bladder and Bowel Problems. Some patients have problems emptying their bladder (urinary retention) and bowels (constipation) or find they cannot control their bladder and bowels (incontinence). Patients with urge incontinence need to urinate frequently or are unable to reach the bathroom before leakage occurs. Bladder problems, and catheterization for urinary retention, can lead to urinary tract infections.
Pain. Most patients have pain at some point during the course of the disease, and many are never completely pain free. MS causes many pain syndromes; some occur for a short time while others continue for a long time. Some worsen with age and disease progression. Pain syndromes associated with MS include trigeminal (facial) pain, powerful spasms and cramps, pressure pain, stiffened joints, and a variety of sensations, including feelings of itching, burning, and shooting pain.
Sexual Dysfunction. Sexual dysfunction is a common problem. Men are likely to have erectile dysfunction, and women often have problems with vaginal lubrication. Sexual dysfunction appears to be highly associated with urinary dysfunction.
Speech and Swallowing Problems. Up to half of patients have trouble chewing or swallowing. Some patients have slurred speech and problems speaking clearly.
Thinking, Concentration, and Memory Problems. Cognitive problems, such as having trouble concentrating, reasoning, and solving problems, affect about half of patients. Up to 75% of patients have problems with memory. These disabilities can create difficulties in the workplace.
Mood Swing.Depression is very common and is sometimes very severe. Depression can be caused both by physical changes in the brain as well as emotional response to the stress of dealing with MS. Less commonly, psychosis (manic depression and paranoia) can occur. About 5% of patients with severe MS have uncontrolled and extreme mood swings where they alternate between uncontrollable laughing and weeping (pseudobulbar affect).
Possible Symptom Triggers
Some patients find that MS flares (relapses) are triggered by certain factors. Possible symptom triggers include.
Infections. Viral and bacterial infections, including urinary tract infections, may provoke MS symptoms.
Heat and Cold. Sudden changes in temperature or humidity can trigger symptoms. Many patients with MS have heat intolerance and find that heat worsens their symptoms.
Stress. Many patients report that stress worsens their symptoms.
Diagnosis
Most patients first seek medical help after an initial attack of symptoms, called a clinically isolated syndrome (CIS). Not all patients who have a CIS go on to develop MS, and it is difficult to predict which patients will or will not.
Multiple sclerosis can be challenging to diagnose as there is no one test for it, and a number of other conditions may mimic its symptoms. To confirm a diagnosis of multiple sclerosis the doctor needs to find:
- Evidence of nerve damage in at least two different areas of the central nervous system (brain, spinal cord, and optic nerves)
- Evidence that the damage occurred in episodes that happened at least one month apart
- No evidence that the damage is caused by other conditions
A diagnosis of multiple sclerosis is based on results from a combination of various tests. These include the patient’s medical history, neurological exam, magnetic resonance imaging (MRI) scans, evoked potential tests, and possibly a spinal fluid test.
Medical History
The doctor will ask about the patient’s personal and family medical history, including lifestyle factors, prescription or other drug use, and other medical conditions that the patient or relatives may have had. The doctor will ask the patient to describe their symptoms, when they occurred, and how long they lasted.
Neurological Exam
In a neurological exam, the doctor will test the patient’s vision and reflexes and evaluate balance, coordination, and muscle strength.
Evoked Potential (EP) Tests
This is a simple and painless electrical test of nerve function that assesses how long it takes nerve impulses from the eye, ear, or skin to reach the brain. It involves having electrodes placed on the scalp over specific areas of the brain that process sensory information. Evoked potential tests can be used to evaluate nerve transmission for vision, sound, or muscle responses in the legs or arms.
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) scans are important diagnostic tools in MS and are used for diagnosing multiple sclerosis, tracking changes over time, and helping to determine treatment effectiveness.
MRIs scans can detect bright patches that indicate areas of damaged myelin and injured tissue (lesions) caused by MS. However, about 5% of people who are confirmed to have multiple sclerosis based on other diagnostic criteria, do not show evidence of lesions in an initial MRI.
Once diagnosed, periodic follow-up MRIs can be used to track the disease and effectiveness of treatments in two ways:
- By distinguishing new lesions from old ones
- Revealing increasing or decreasing numbers of lesions within the central nervous system over time
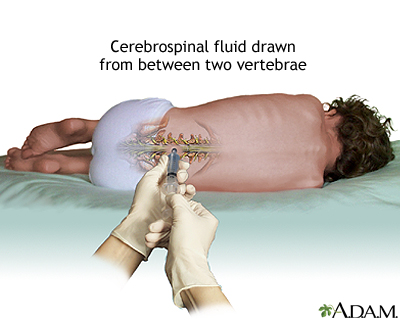
Cerebrospinal Fluid Analysis
A spinal fluid test by itself cannot confirm or exclude multiple sclerosis but it can be useful when combined with other tests. Obtaining a sample of spinal fluid requires a lumbar puncture (also called spinal tap). Spinal fluid in patients with MS usually contains unusually high levels of immunoglobulin G (IgG) antibodies as well as other proteins and fragments of myelin. These can be signs of an autoimmune disorder, but not necessarily multiple sclerosis.
Ruling Out Other Disorders
The symptoms of MS overlap with a number of other diseases that must be ruled out. These conditions include stroke, alcoholism, emotional disorders, Lyme disease, chronic fatigue syndrome, fibromyalgia, AIDS, cervical spondylosis, certain neurologic degenerative illnesses, transverse myelitis, and certain other autoimmune disorders (hypothyroidism, scleroderma, Sjogren syndrome, vasculitis, and systemic lupus erythematosus).
Treatment
The goals of treatment for multiple sclerosis are:
- Modify the disease course by reducing the number and severity of relapses (also called exacerbations or flares), reducing accumulation of lesions, and slowing the progression of disability
- Treat relapses on a short-term as-needed basis
- Manage symptoms
Patients are recommended to seek care from a neurologist experienced in treating multiple sclerosis.
Early Treatment. Evidence strongly suggests that the most destructive changes from multiple sclerosis in the brain occur very early on in the disease process -- and may cause considerable damage even before symptoms begin.
Many doctors recommend treatment after a first neurological episode of MS (a clinically isolated syndrome) using disease-modifying drugs. The best current approach is to use specific findings from MRI scans to determine patients at highest risk for progression, making them likely candidates for early treatment with these drugs.
Over a third of patients will progress even with immediate treatment, but without early treatment about half of patients will progress to clinically identifiable multiple sclerosis.
Treatment with Disease-Modifying Drugs
Eight disease-modifying drugs are approved by the FDA for treatment of multiple sclerosis:
- Interferon beta-1b (Betaseron, Extavia). Given in subcutaneous (under the skin) injections every other day.
- Interferon beta-1a (Avonex). Given as weekly intramuscular injections.
- Interferon beta-1a (Rebif). Given in subcutaneous injections three times a week.
- Glatiramer acetate (Copaxone). Given daily in subcutaneous injections.
- Natalizumab (Tysabri). Given by intravenous infusion once every four weeks.
- Mitoxantrone (Novantrone). Given intravenously once every three months for 2 - 3 years at most.
- Fingolimod (Gilenya). Taken daily as a pill.
- Teriflunomide (Aubagio). Taken daily as a pill.
The interferon drugs (Betaseron, Extavia, Avonex) and glatiramer acetate (Copaxone) are given by self-injection at home. Natalizumab (Tysabri) and Mitoxantrone (Novantrone) are given by injection in a hospital or medical clinic setting.
Most of these drugs are taken on a long-term basis. They can help reduce disease activity and progression for relapse-remitting MS (the most common form of MS) and other types of MS that have relapses (secondary-progressive MS, progressive-relapsing MS). At this time, there are no proven treatments for primary-progressive MS. Disease-modifying drugs can have significant side effects.
If disease-modifying drugs do not work, doctors may try other drugs that are not specifically approved for MS. They include intravenous immunoglobulin (IVIg), methotrexate, azathrioprine (Imuran), and cyclophosphamide (Cytoxan).
Treating Acute Relapses
A relapse (also called exacerbation or flare-up) is an attack that brings about new symptoms or worsening of old symptoms. It is caused by inflammation in the central nervous system. Relapses can be mild or severe. They may last from a few days to several months.
Pseudoexacerbations are temporary worsening of symptoms that are usually caused by an external trigger, such as infection, heat, or stress. Pseudoexacerbations do not involve myelin inflammation and symptoms usually subside within 24 hours. To be considered a true relapse or exacerbation, symptoms and neurological signs must last at least 24 hours and occur at least 30 days after a previous attack.
Not all acute relapses require treatment. For attacks that are severe, a short course of high-dose corticosteroid drugs is the standard treatment. Typically, intravenous methylprednisolone (IVMP) is given for 3 - 5 days. Sometimes this is followed by oral prednisolone for a few days. Long-term treatment with corticosteroids is not recommended. These drugs can cause serious side effects and do not have any effect on MS disease progression.
Other treatment options for relapses are injections of adrenocorticotropic hormone (ACTH) and plasmapheresis (plasma exchange). These treatments are usually reserved for a small percentage of patients with very severe symptoms who do not respond to steroid drugs.
Treating Symptoms
MS symptoms are managed through a combination of treatment approaches that include medications, self-care, and physical and occupational therapy.
Drugs that are specifically approved for treating symptoms associated with MS include:
- Dalfampridine (Ampyra) is approved to improve walking in patients with MS. In clinical trials, patients treated with dalfampridine had faster walking speeds than patients who received placebo. Dalfampridine is taken as a pill. The drug may cause seizures.
- Onabotulinumtoxin A (Botox) is approved to treat upper limb spasticity in the flexor muscles of the elbow, wrist, and fingers. It is also approved to treat urinary incontinence associated with certain neurological conditions, including multiple sclerosis. Botox is given by injection.
- Baclofen (Lioresel, Gablofen) is approved to treat severe spasticity. It is given orally in pill form, and for severe spasticity it can be injected intrathecally (into the spinal fluid) through a surgically implanted pump.
Investigational Treatments
Oral Medications. There are numerous new drugs for multiple sclerosis that are currently being investigated in clinical trials. Among them are several new oral medications, including dimethyl fumarate (BG-12), a pill for relapse-remitting MS that has shown promise in phase III trials.
Stem Cell Transplantation. Investigators are studying the benefits of stem cell transplantation procedures. Stem cells are produced in the bone marrow and are the early forms for all blood cells in the body (including red, white, and immune cells). Early studies indicate that stem cell transplantation may slow MS progression. Larger randomized controlled trials are currently under way.
Unproven Treatments. Patients with MS should be highly skeptical about treatments touted for MS that are unproven, have not been rigorously investigated, and have scant scientific evidence for safety or efficacy. In 2012, for example, the FDA warned of injuries and deaths associated with “liberation therapy.” This surgical procedure uses balloon angioplasty devices or stents to widen veins in the chest and neck associated with chronic cerebrospinal venous insufficiency (CCSVI). However, there is no proven link between CCSVI and MS. The procedure has caused stroke, nerve damage, and death in several patients.
Medications
Interferon Beta Drugs
Interferons (so-called because they “interfere” with viral replication) suppress inflammatory factors in the immune system that are associated with the attack on myelin. Interferon drugs are the main treatments for relapsing-remitting MS. Doctors recommend that these medications be used early in the course of the disease and continued indefinitely, unless they produce no benefits or have severe side effects. When the drug is discontinued, disease activity may increase.
Brands. Interferon drugs used for MS are interferon beta-1b (Betaseron, Extavia) and interferon beta-1a (Avonex, Rebif). Avonex and Rebif are the same chemical, but Avonex is given as weekly intramuscular injection and Rebif is taken as subcutaneous injections three times a week.
Side Effects. Side effects include:
- Flu-like symptoms. Flu-like symptoms (fever, chills, sweating, muscle aches, and fatigue) following injection are a common complaint. These symptoms usually lesson over time. Taking acetaminophen (Tylenol) before the injection can help.
- Injection site reactions. Pain, redness, and swelling can occur at the injection site. Applying ice or a cool compress to the skin a few minutes before injection can help prevent pain.
- Less common side effects include allergic reactions, depression, mild anemia, low white blood cell counts, and liver abnormalities. Patients who take interferon drugs should have a baseline liver function test at the start of treatment and receive periodic testing afterwards. Patients should avoid alcohol.
Neutralizing Antibodies That Reduce Effectiveness. Over time, people taking interferons develop antibodies to the drugs, some of which can neutralize their effects. The risk for neutralizing antibodies (NAbs) increases with higher doses and greater frequency of use. Interferons injected under the skin (Betaseron, Rebif) are more likely to produce neutralizing antibodies than Avonex, which is injected into a muscle. Patients who have this reaction may be treated with an alternative interferon or with glatiramer, which has an extremely low risk, for NAbs. Often after switching drugs, NAb levels decline, and the patient may be able to return to the original interferon.
Glatiramer Acetate (Copaxone)
Glatiramer acetate (Copaxone) is a synthetic molecule that resembles a basic protein found in myelin. It is used as a decoy to trick white blood cells into attacking it instead of myelin. It is approved to help reduce the frequency of relapses in patients with relapse-remitting MS. It comes in pre-filled syringes and is given by subcutaneous (under the skin) injections.
The best results are for patients in early stages of MS, but the longer patients remain on the drug, the greater the improvement. Benefits have lasted for years. Glatiramer acetate appears to work as well as interferon beta drugs in decreasing the number and severity of relapses, but it may have less effect on lesions.
Side Effects. Side effects may occur right after the injection. They include pain at the injection site, chest pain, rapid heartbeat, flushing, anxiety, and shortness of breath.
Natalizumab (Tysabri)
Natalizumab (Tysabri) is a monoclonal antibody drug approved for treatment of MS. Monoclonal antibodies (MAbs) are drugs that target specific antibodies involved with the immune response. Natalizumab should only be taken by patients who have not responded to or who cannot tolerate other disease-modifying drugs. Natalizumab can only be taken alone, not in combination with other immune-modifying drugs. The drug is given by IV infusion once every 4 weeks.
Many patients who take natalizumab have reported great improvement in reduction of MS relapses and the drug appears to work well in slowing disease progression. However, natalizumab does carry risks for potentially serious side effects.
Common Side Effects. Common side effects include headache, fatigue, depression, joint pain, abdominal and chest discomfort, along with urinary tract, pneumonia, and other infections.
Severe Side Effects. Patients who take natalizumab must be monitored for signs of progressive multifocal leukoencephalopathy (PML). PML is a rare neurological disease that can affect people with compromised immune systems. As of 2012, PML had occurred in several hundred patients out of nearly 100,000 treated with the drug.
The risk for PML appears to increase when patients receive more than 24 natalizumab infusions (2 years of treatment). The risk is also increased if a patient has previously used an immunosuppressant drug (such as mitoxantrone, azathioprine, methotrexate, cyclophosphamide, or mycophenolate). Natalizumab should not be given in combination with another immunomodulatory medication, and patients with compromised immune systems should not receive this drug. The risks for PML are reduced when natalizumab is given alone and not in combination with other immunomodulatory medications.
Patients who take natalizumab must enroll in a special program called TOUCH, which is run by the drug's manufacturer. Patients need to get magnetic resonance imaging (MRI) brain scans before they begin taking the drug, and they are evaluated regularly during drug treatment to make sure they are not at risk of developing PML. Symptoms of PML may include progressive weakness on one side of the body, clumsiness of limbs, vision disturbances, and changes in thinking, memory, and orientation that may result in confusion or personality changes.
Natalizumab may also cause liver abnormalities. Cases of liver injury have been reported within a week after a first dose of natalizumab, as well as after multiple doses. Signs of liver injury include yellowing of skin and eyes (jaundice), sudden darkening of urine, fatigue, and nausea and vomiting. Patients should immediately contact their doctors if any of these symptoms develop. If blood tests confirm liver injury, natalizumab should be discontinued.
Mitoxantrone (Novantrone)
Mitoxantrone (Novantrone) was the first drug approved specifically for secondary-progressive MS and progressive-relapsing MS. It is also used to treat worsening relapsing-remitting MS. Mitoxantrone is often used in combination with an interferon beta drug or glatiramer acetate (Copaxone).
Cumulative doses can have toxic effects on the heart, including heart failure, so the drug is only used for a limited period of 2 - 3 years. Patients should have a heart evaluation, including evaluation of left ventricular ejection fraction (LVEF), before starting this drug and before each dose administration. Mitoxantrone can also increase the risk for leukemia.
Side Effects. In addition to risks of heart damage, common side effects of mitoxantrone include nausea, thinning hair, bladder infections, mouth sores, and loss of menstrual periods. Patients may experience a temporary bluish color to their urine or eyes during the first 24 hours after IV infusion.
Fingolimod (Gilenya)
Fingolimod (Gilenya) was the first oral drug approved to treat relapsing forms of MS. Approved in 2010, fingolimod may help reduce relapses (flare-ups) and delay disability progression. Fingolimod is taken daily as a pill.
Fingolimod is the first in a new class of drugs called sphingosine 1-phosphate receptor (S1PR) modulators. It is thought to work by keeping white blood cells (lymphocytes) contained to the lymph nodes and preventing them from traveling to the central nervous system where they could damage the myelin that protects nerve fibers.
Common Side Effects. Common side effects may include headache, influenza, diarrhea, back pain, cough, and abnormal liver enzymes.
Severe Side Effects. Fingolimod can decrease heart rate (bradycardia, or bradyarrythmia) especially after the first dose. (The doctor should monitor the patient for six hours after the first dose to make sure there are no heart problems.) Heart rates usually stabilize within a month after starting fingolimod. Because of these cardiac side effects, patients with certain pre-existing heart conditions or recent history of heart attack or stroke should not use fingolimod.
Other serious side effects include macular edema (fluid accumulation in a part of the eye’s retina), increased risk of serious infections, and shortness of breath. Fingolimod can cause liver problems; patients should have liver tests before starting the drug. This drug may cause birth defects. Women of childbearing age who take fingolimod should use birth control while on the drug.
Teriflunomide (Aubagio)
In 2012, the FDA approved teriflunomide (Aubagio), the second oral treatment for relapsing forms of MS. Like fingolimod, teriflunomide is taken as a once-daily pill. Heath risks for liver problems and birth defects are similar to those of fingolimod.
Lifestyle Changes
People with multiple sclerosis should make every effort to preserve their general health. A healthy diet, sufficient rest, establishing priorities to conserve energy, and developing emotional support networks can all be very helpful.
Rehabilitation Therapies
Patients with MS can benefit from various rehabilitation services to help them cope with the physical and emotional symptoms of their condition.
Physical Therapy. Physical therapists can provide professional guidance on exercise programs. Patients with MS should engage in a variety of exercises including stretching, muscle strengthening, and range-of-motion. Exercise can help reduce fatigue and relieve muscle spasticity. Physical therapists can also provide advice on how to best use mobility aids (such as canes, crutches, and scooters) and other assistive devices.
Occupational Therapy. Occupational therapists help patients learn how to improve their functioning and independence within their home and workplace environments. They can provide professional advice on what sort of adaptive tools, such as grab bars, should be used in the bathroom, bedroom, and kitchen. Occupational therapists may also be able to evaluate and treat problems with thinking and memory.
Vocational Therapy. Vocational therapists provide guidance on how patients can best manage in the workplace.
Speech/Language Therapy. Speech and language therapists treat problems with speech and communication. They can also help address problems with swallowing.
Psychological Therapies. Psychotherapy can help patients and their families cope with a difficult and chronic disease.
Dietary Factors
Some dietary suggestions for patients with MS include:
- Drink 2 quarts of water a day. Drinking water helps avoid constipation (although it may cause difficulties in patients who also have urge incontinence).
- Eat a diet rich in fiber, particularly from whole grains (especially bran, oats, or flax), fruits (particularly prunes), and vegetables.
- Low-fat diets have not proven to have much effect on MS but are, in any case, generally healthy.
- Omega-3 fatty acids, found in oily fish and fish oil supplements, have been associated with protection against inflammation and some reduction in symptoms in people with various autoimmune conditions. Such fatty acids are also available in supplements as docosahexaenoic (DHA) and eicosapentaenoic (EPA) acids. Standards for optimal amounts and forms of omega-3 fatty acids have not yet been established, however. Some doctors recommend that people with MS eat three fish meals a week.
Special diets, such as those that are gluten- or yeast-free, do not have any direct effect on the symptoms or course of MS.
Temperature Control
Body overheating causes demyelinated nerves to function less efficiently than usual. Although this effect is resolved within a few hours of regaining normal body temperature, active cooling can help reduce fatigue and improve stability.
The following measures may help:
- Use air conditioners in the summer.
- Keep the home slightly cool in winter.
- Avoid swimming in heated pools. However, swimming is an excellent exercise as water supports the body and cool water dissipates heat.
Prevention of Influenza
MS symptoms worsen during a cold or the flu, probably because of increased immune system activity. Doctors recommend that patients with MS receive a flu shot in the fall. However, patients should not take the nasal spray version of the flu vaccine (FluMist Intranasal). Unlike the flu injection vaccine, which uses an inactivated virus, FluMist contains a live virus. Live virus vaccinations may be harmful for people with MS, especially those who take immune-suppressing drugs.
Complementary and Alternative Medicine
Many patients with MS try some form of nontraditional remedies. Research on any benefits is slim, and there may be some danger with certain herbal remedies. Alternative therapies used to treat MS include:
Relaxation and Meditation Techniques. Patients may try relaxation, meditation, biofeedback, music therapy, yoga, tai chi, and massage therapy. They are generally harmless, and possibly helpful.
Acupuncture. Some patients report benefit from acupuncture.
Herbs and Supplements
Generally, manufacturers of herbal remedies and dietary supplements do not need FDA approval to sell their products. Just like a drug, herbs and supplements can affect the body's chemistry, and therefore have the potential to produce side effects that may be harmful. There have been a number of reported cases of serious and even lethal side effects from herbal products. Patients should check with their doctor before using any herbal remedies or dietary supplements
The following warnings are of particular importance for people with multiple sclerosis:
- Antioxidants. Some patients use antioxidant vitamins or supplements, since the destruction in the MS disease process may be partly due to oxidation (chemical damage from particles called oxygen-free radicals). Theoretically, however, antioxidants can trigger T cells and macrophages (inflammatory components of the immune system) and, therefore, may pose some danger to patients. Small studies to date have not found any worsening of the disease from taking vitamin supplements, but patients should be cautious. No vitamins studied for MS, including carotenoids, vitamin C, vitamin E, B12 injections, or vitamin D, have been proven to be beneficial.
- Gingko. Although the risks for gingko appear to be low, there is an increased risk for bleeding at high doses. Ginkgo can also interact with high doses of vitamin E, anti-clotting medications, aspirin, and NSAIDs. Large doses may cause convulsion. Randomized trials have failed to show any benefit.
- Bee Venom. For years, anecdotal reports have claimed that bee stings relieve some MS symptoms. No studies have confirmed any benefits. Bee venom contains many chemicals, some of which can cause severe and sometimes deadly allergic reactions in some people.
- Linoleic Acid. Linoleic acid, commonly known as evening primrose oil, is a polyunsaturated fatty acid believed by some people to be helpful because myelin is composed of fatty acids. No study has proven that it is beneficial, but supplements sold in health food stores do not appear to be harmful.
Resources
- www.ninds.nih.gov -- National Institute of Neurological Disorders and Stroke
- www.aan.com -- American Academy of Neurology
- www.msassociation.org -- Multiple Sclerosis Association of America
- www.nationalmssociety.org -- National Multiple Sclerosis Society
- www.msfacts.org -- Multiple Sclerosis Foundation
- www.abledata.com -- National database of assistive devices and rehabilitation equipment
References
Calabresi P. Multiple sclerosis and demyelinating conditions of the central nervous system. In: Goldman L, Ausiello D, eds. Cecil Medicine. 24th ed. Philadelphia, Pa: Saunders Elsevier; 2012:chap 419.
Farinotti M, Simi S, Di Pietrantonj C, McDowell N, Brait L, Lupo D, Filippini G. Dietary interventions for multiple sclerosis. Cochrane Database Syst Rev. 2007 Jan 24;(1):CD004192.
Goodin DS, Cohen BA, O'Connor P, Kappos L, Stevens JC; Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Assessment: the use of natalizumab (Tysabri) for the treatment of multiple sclerosis (an evidence-based review): report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2008 Sep 2;71(10):766-73
International Multiple Sclerosis Genetics Consortium, Hafler DA, Compston A, Sawcer S,Lander ES, Daly MJ, et al. Risk alleles for multiple sclerosis identified by a genomewide study. N Engl J Med. 2007 Aug 30;357(9):851-62. Epub 2007 Jul 29.
Kappos L, Freedman MS, Polman CH, Edan G, Hartung HP, Miller DH, et al. Effect of early versus delayed interferon beta-1b treatment on disability after a first clinical event suggestive of multiple sclerosis: a 3-year follow-up analysis of the BENEFIT study. Lancet. 2007 Aug 4;370(9585):389-97.
Kappos L, Radue EW, O'Connor P, Polman C, Hohlfeld R, Calabresi P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010 Feb 4;362(5):416-26. Epub 2010 Jan 20.
Khan F, Ng L, Turner-Stokes L. Effectiveness of vocational rehabilitation intervention on the return to work and employment of persons with multiple sclerosis. Cochrane Database Syst Rev. 2009 Jan 21;(1):CD007256002819.
Kuehn BM. FDA warns about the risks of unproven surgical therapy for multiple sclerosis. JAMA. 2012 Jun 27;307(24):2575-6.
Lovera J, Bagert B, Smoot K, Morris CD, Frank R, Bogardus K, et al. Ginkgo biloba for the improvement of cognitive performance in multiple sclerosis: a randomized, placebo-controlled trial. Mult Scler. 2007 Apr;13(3):376-85. Epub 2007 Jan 29.
Marriott JJ, Miyasaki JM, Gronseth G, O'Connor PW; Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Evidence Report: The efficacy and safety of mitoxantrone (Novantrone) in the treatment of multiple sclerosis: Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2010 May 4;74(18):1463-70.
Multiple Sclerosis Therapy Consensus Group (MSTCG), Wiendl H, Toyka KV, Rieckmann P, Gold R, Hartung HP, et al. Basic and escalating immunomodulatory treatments in multiple sclerosis: current therapeutic recommendations. J Neurol. 2008 Oct;255(10):1449-63. Epub 2008 Oct 29.
Pelletier D, Hafler DA. Fingolimod for multiple sclerosis. N Engl J Med. 2012 Jan 26;366(4):339-47.
Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011 Feb;69(2):292-302. doi: 10.1002/ana.22366.
Prakash RS, Snook EM, Lewis JM, Motl RW, Kramer AF. Cognitive impairments in relapsing-remitting multiple sclerosis: a meta-analysis. Mult Scler. 2008 Nov;14(9):1250-61. Epub 2008 Aug 13.
Ransohoff RM. Natalizumab for multiple sclerosis. N Engl J Med. 2007 Jun 21;356(25):2622-9.
|
Review Date:
9/25/2012 Reviewed By: Harvey Simon, MD, Editor-in-Chief, Associate Professor of Medicine, Harvard Medical School; Physician, Massachusetts General Hospital. Also reviewed by David Zieve, MD, MHA, Medical Director, A.D.A.M., Inc. |